‘Best early birthday present’: 90-year-old Briton becomes first person to be given Pfizer COVID-19 vaccine – Health News , Firstpost
[ad_1]
‘I feel so privileged to be the first person vaccinated against COVID-19,’ said Margaret Keenan, who turns 91 next week
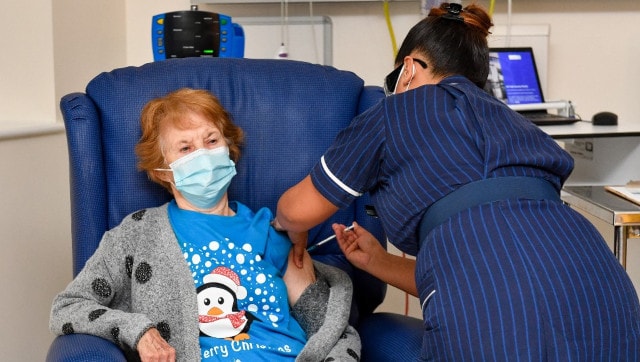
Nurse May Parsons administers the Pfizer-BioNtech COVID-19 vaccine to Margaret Keenan at Coventry, England. AFP
A 90-year-old grandmother, Margaret Keenan and an 81-year-old from Warwickshire named — wait for it — William Shakespeare were the first two people who were administered the COVID-19 vaccine produced by Pfizer-BioNTech.
This marked the start of the biggest vaccination programme in the history of Britain.
Both Keenan and Shakespeare were administered the vaccines at a hospital in Coventry, central England.
Second patient to get the COVID jab at University Hospital Coventry – would you believe it….William Shakespeare from Warwickshire pic.twitter.com/y0LzxgbJ9w
— Hugh Pym (@BBCHughPym) December 8, 2020
“I feel so privileged to be the first person vaccinated against COVID-19 ,” Keenan, who turns 91 next week was quoted as saying by AFP.
Wearing a face-mask and sporting a snowman T-shirt under a grey cardigan she told reporters: “It’s the best early birthday present I could wish for because it means I can finally look forward to spending time with my family and friends in the New Year after being on my own for most of the year.”
‘We’ll beat this together’, says Boris Johnson
Prime Minister of the United Kingdom Boris Johnson hailed the news of the first vaccinations against COVID-19 and said —
Today the first vaccinations in the UK against COVID-19 begin. Thank you to our NHS, to all of the scientists who worked so hard to develop this vaccine, to all the volunteers – and to everyone who has been following the rules to protect others. We will beat this together. https://t.co/poOYG1vHQe
— Boris Johnson (@BorisJohnson) December 8, 2020
The Telegraph quoted Kate Bingham, the chairman of the Government’s vaccine task force, as saying, “It was a daunting challenge to try to deliver on the goals set by the Prime Minister. We did deliver on the goals, which was to protect the UK as soon as possible with vaccines and I’m delighted today is V-Day.”
She was further reported to have said, “”This is a monumental challenge to do a mass vaccination of adults – it’s never been done before. But the feedback we have had is that the UK is probably the most well-prepared country in the world to receive and deploy these vaccines.”
UK Health Secretary Matt Hancock, who has offered to have the jab on live television to allay public fears, said the rollout was a “key moment” that would protect the most vulnerable, AFP reported.
The head of the state-run National Health Service in England, Simon Stevens, said it was a “decisive turning point” against the “greatest health challenge” since the NHS was founded in 1948.
Elderly people, healthcare workers to get first jabs
The first in line to get the vaccine on what has been dubbed “V-Day” are the over-80s, care home workers and at-risk frontline health and social care staff.
They will then require a second jab in 21 days’ time.
Last week Britain became the first country to approve the Pfizer-BioNTech vaccine, raising hopes of a breakthrough in the pandemic, which has killed more than 1.5 million people worldwide.
Britain has been one of the worst-affected countries in the world, with more than 61,000 deaths in the outbreak from 1.6 million cases.
Regulatory approval for the vaccine was given last Wednesday, sparking a race against time to prepare scores of vaccination centres across the country.
The UK has ordered 40 million doses of the jab — enough to vaccinate 20 million people — with 800,000 in the first batch.
Up to four million doses are expected by the end of December.
Queen could lead way
The mass vaccination drive is a coordinated response by all four nations of the UK — England, Scotland, Wales and Northern Ireland — which normally set their own health policies.
The public has been largely favourable to the rapid approval of the vaccine, but ministers and health professionals are aware they still need to combat mistrust.
The independent Medicines and Healthcare products Regulatory Agency maintains that no corners were cut and its assessment and approval procedures met stringent international norms.
NHS England said thousands had already been given the jab during trials with no serious side effects.
Nevertheless, it has been reported Queen Elizabeth II, who at 94 is among those first in the line for the vaccination because of her age, could front a public awareness campaign urging compliance.
The government said it will hand out vaccine cards to remind people to get the booster after three weeks, but insisted it was not introducing immunity certificates.
‘Marginal impact’ in winter’
The chief medical officers of England, Scotland, Wales and Northern Ireland said the vaccine will as a result only have a “marginal impact” on hospital numbers over the winter months.
Johnson called for patience and urged the public to stick to strict social distancing guidelines to prevent a spike in cases, particular as rules are relaxed over Christmas.
Health officials have already run into a logistical headache about how to administer the vaccine to elderly or infirm care home residents.
The vaccine needs to be stored at -70 degrees Celsius (-94 Fahrenheit), leaving hospitals and other medical hubs as the only places able to deal with such ultra-low temperatures.
With the Pfizer-BioNTech drug made in Belgium, concerns have also been raised about potential disruption to supply when Britain leaves the European Union’s single market and customs union.
But the UK government said the military is on stand-by to air-lift the vaccine if there is any border disruption from January 1.
The bulk of Britain’s vaccine requirements are expected to be met by a jab developed by AstraZeneca and the University of Oxford, which is awaiting regulatory approval.
The government has ordered an initial 100 million doses of the drug, which is cheaper to manufacture, and easier to store and transport using conventional fridges.
With inputs from AFP
[ad_2]

Comments are closed.